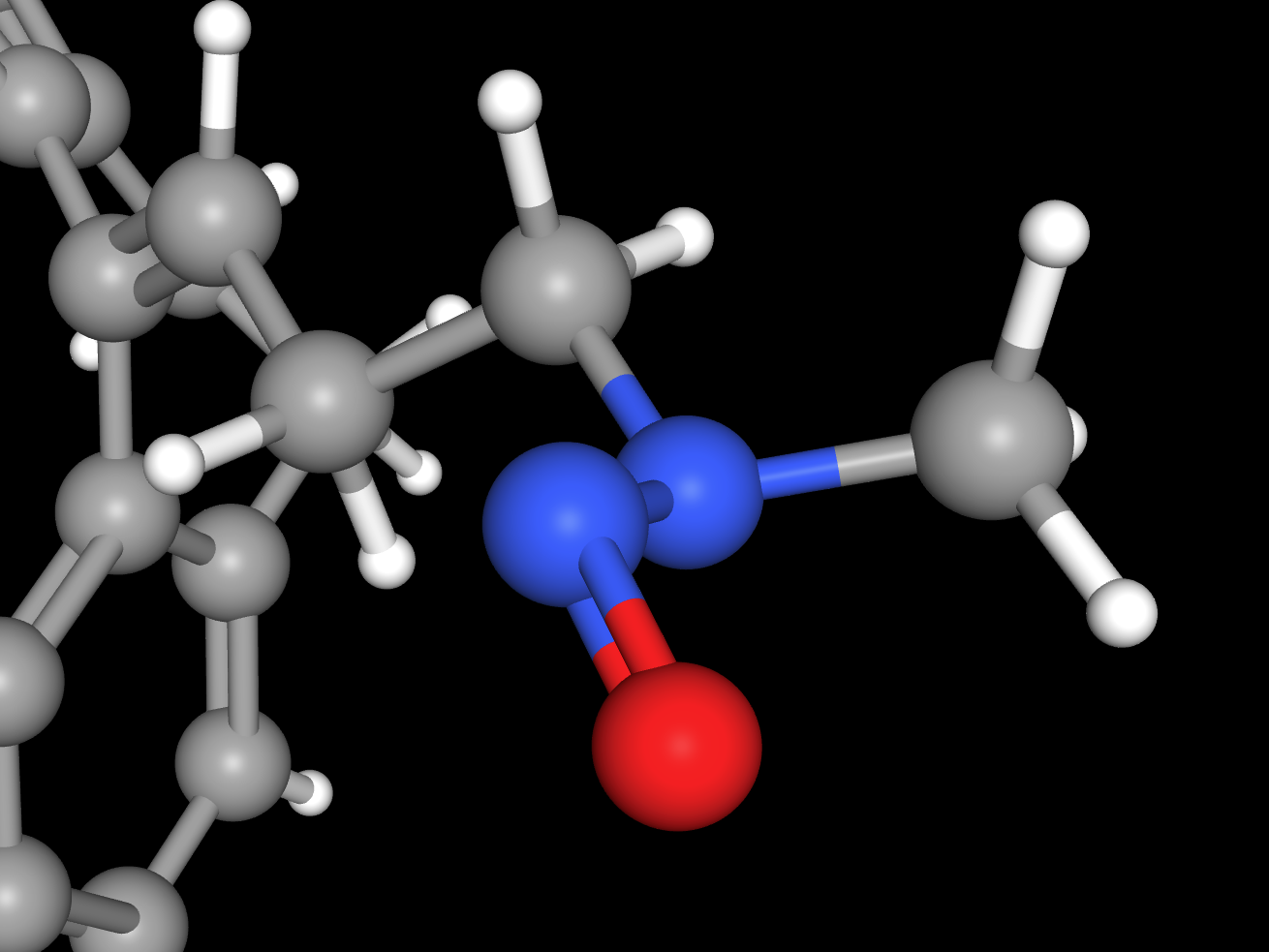
Addressing the challenge of nitrosamine contamination demands a precisely customised approach to risk assessment, analytical testing, and mitigation. A one-size-fits-all solution simply is not effective.

Chemika is thrilled to announce a significant capacity increase at our Girraween site, set to be finalized early in 2026.
This expansion comes through the successful refit and conversio...

Richard Miller has returned to Chemika following a year travelling the world.
Richard will be resuming his role as Laboratory Services Manager and will become your primary point of cont...


Chemika will be exhibiting at the PDA Aseptic Manufacturing Excellence Conference on October 13-14, 2025.
Our Managing Director, Alan Doughty and Laura Piva will be at our booth to disc...

Chemika’s Analytical Development Manager, Andrew Jones, and Managing Director, Alan Doughty, attended the BIO International Convention 2025 in Boston in June.
We were proud to be part...

We’re thrilled to announce that Alan Doughty (Managing Director & Chief Chemist)

This year is EXTRA special for Chemika as we proudly celebrate 25 years of excellence in chemical testing! Since our journey began on July 1, 2000, we’ve been on an incredible trajectory – growing, evolving, and shaping the industry.<...

Chemika has expanded its leachables and extractables testing services with the recent commissioning of a triple quadrupole LCMS system.
We have been conducting extractabl...

Are material releases being delayed while your testing laboratory completes investigations?
Laboratory reporting times play a crucial role in the timely approval of incoming goods and t...